The Independent Center for Integrative Education: Learning without Limits
1/30/11
The topic of our sixth class was . Please read the summary below and the homework assignment at the bottom.
There are many different types of atoms we are dealing with when cleaning water. Water itself consists of oxygen and hydrogen, which makes these two elements exceptionally important. We also talked about salts, acids, and bases, which contain such atoms as sodium (Na) and chlorine (Cl). Now, it is time to introduce one more element, whose name is nitrogen (N). This element is No. 7 in the Periodic Table, after hydrogen (H), helium (He), lithium (Li), beryllium (Be), boron (B) and carbon (C). Nitrogen is followed by oxygen (O). A nitrogen atom is 14 times heavier than a hydrogen atom.
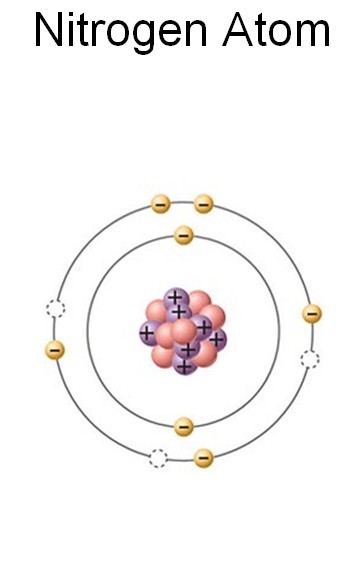
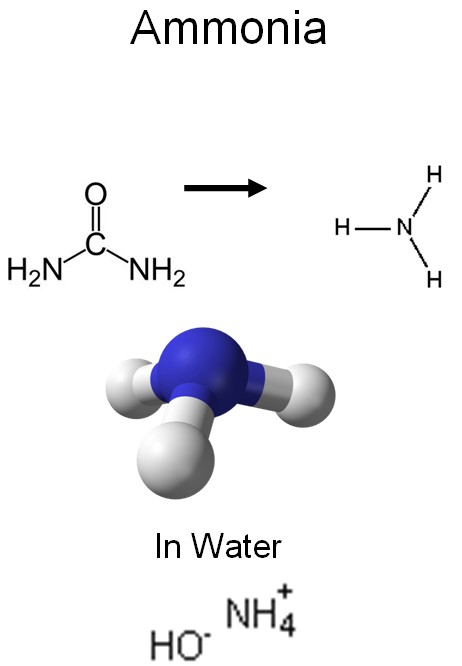
A nitrogen atom has seven electrons. Five of them belong to the outermost shell (orbit), which is missing three more electrons to be full. Therefore, a nitrogen atom might steal electrons from three nearby hydrogen atoms to fill up the outermost shell. This would produce ammonia (NH3). Ammonia can easily dissolve in water. The resulting solution is also called ammonia. We will have to find a good way to clean water from ammonia.
Where does ammonia comes from when it pollutes water? The main source of it is urine, which contains a chemical compound called urea (NH2)2CO)
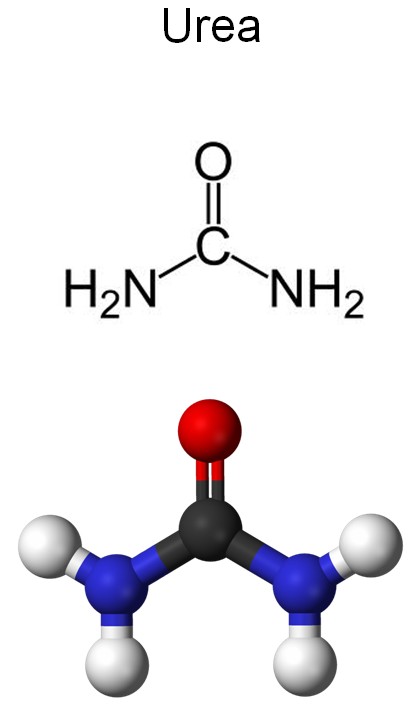
Urea is not very stable in water and easily breaks down, producing ammonia and carbon dioxide:
(NH2)2CO + H2O → 2NH3 + CO2
When cleaning water from urea and/or ammonia, we need to remember, that the nitrogen that they contain plays an extremely important role in the life of any living creature, us included. Indeed, nitrogen is one of the major components of proteins, which are the "building blocks of life."
Proteins may be just molecules, but they are large and complex. If molecules with which we have already met, such as water, carbon dioxide, urea, or ammonia contain only a few atoms each, a protein molecule may easily contain a few hundred thousand atoms each.
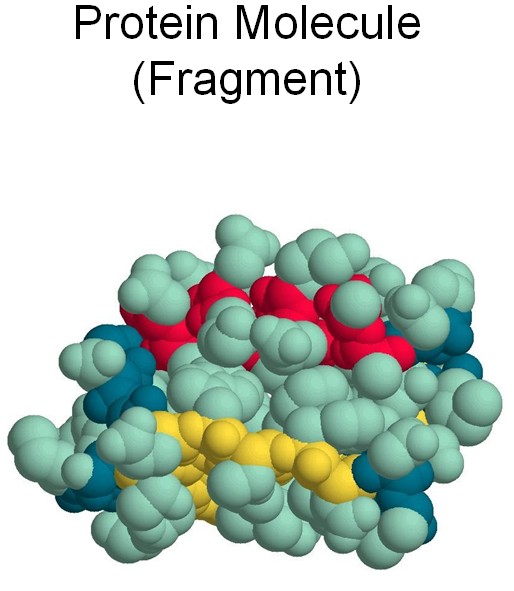
Proteins are built of amino acids that can connect to each other, producing long chains.
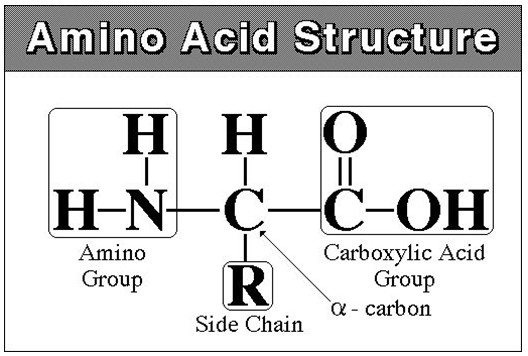
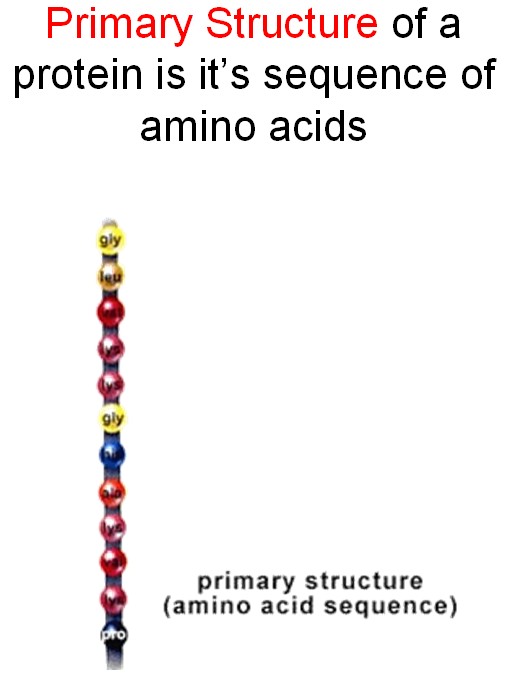
The chain does not stay straight. Attraction among amino acids would bend it in a way specific for each protein.
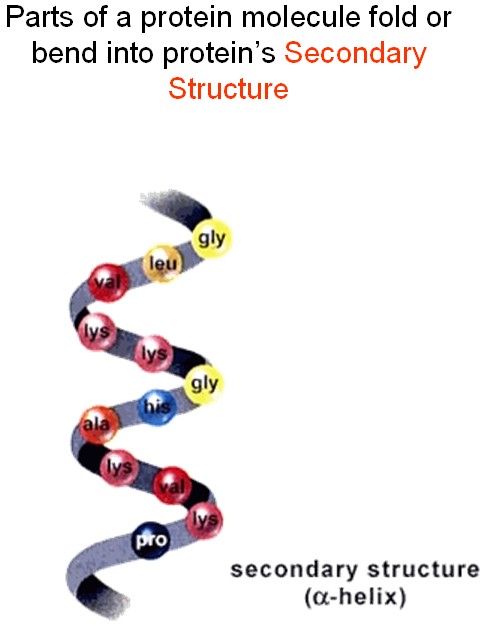
The folded chain twists and folds further due to attraction between different parts of the folds.
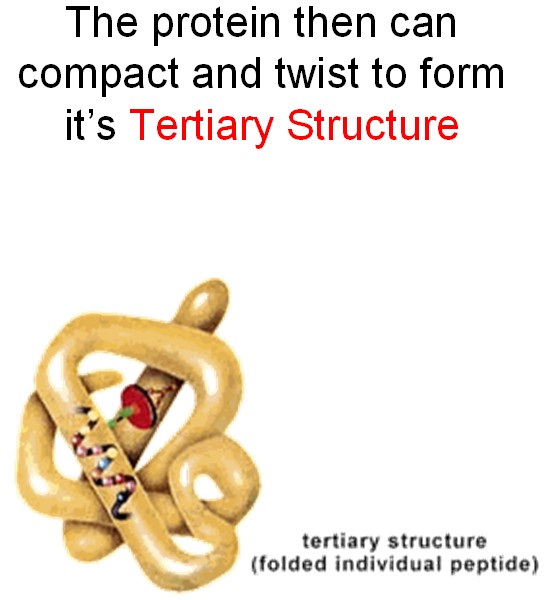
The proteins are team players. Usually, they collaborate, working together to achieve a biologically important goal. Sometimes, they even fold together, producing a huge molecule that, in fact, consists of a few proteins that are unseparable (try to untie a fishing line after it gets tangled and you will see why).
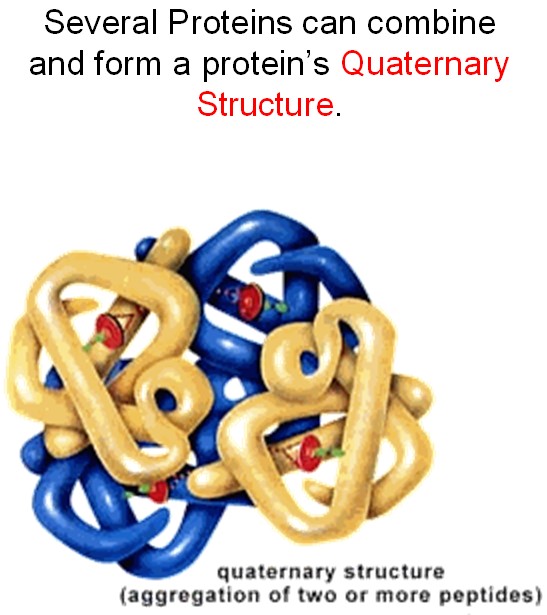
Because nitrogen is so important, we cannot just dispose of the nitrogen after cleaning water from ammonia. It should absolutely be recycled.
Actually, nitrogen is one of the most abundant elements around us. About four fifths of our atmosphere is nitrogen (the other fifth being oxygen. Other gases, such as water vapor, carbon dioxide, argon, etc. take up less than 2%). The problem is, the atmospheric nitrogen is almost impossible to use. In the atmosphere, nitrogen atoms are usually present in pairs that form the nitrogen molecule N2. Those molecules are extremely strong and do not enter any chemical reactions. They are practically as inert as noble gases such as helium or argon. It takes huge temperatures such as the one in a lightning bolt, to split the nitrogen molecule, making it chemically active. That is because two nitrogen atoms share not one, not two, but three electrons that keep the atoms together. On Earth, chemically active nitrogen is mostly recycled in nature, and we will have to recycle it in our Lunar station.
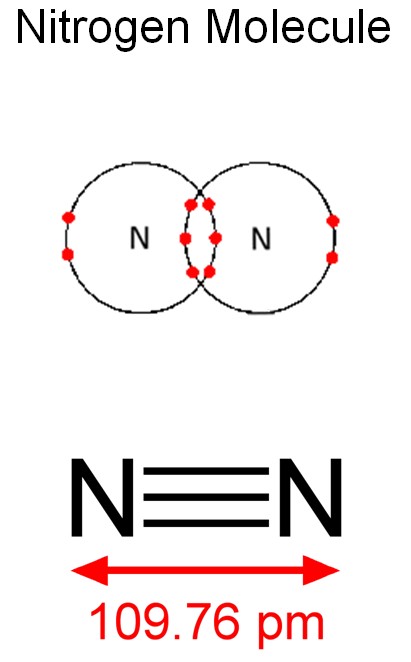
(pm here means picometer, which is 10-12, or one millionth of one millionth of a meter)
HOMEWORK ASSIGNMENT:
Next time, we are going to have a field trip to the United Water water cleaning facility where water is taken from a natural reservoir and turned into drinking water by sedimentation, filtering, and chemical treatment. Please read about the United Water at http://www.unitedwater.com/environment.aspx.
Dear parents, I would highly encourage you to help you children with this assignment.