The Independent Center for Integrative Education: Learning without Limits
12/30/2010
The topic of our fourth class was how to clean water from salts. Please read the summary below and the homework assignment at the bottom.
We discussed in more details two processes that may produce fresh water from salty water, namely distillation and zone freezing.
Distillation is a process in which water evaporates and then condenses in another place. When salty water evaporates, the salt stays behind, so condensed (distilled) water is virtually free of salt.
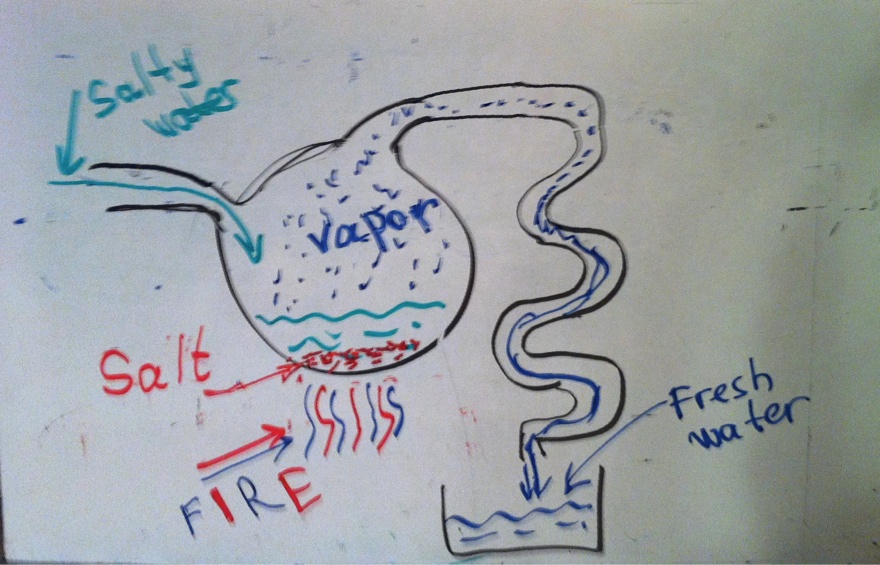
Usually, the purpose of distillation is to collect the clean water and dispose of the salt. If the goal is to collect the salt from sea water, the evaporated water is not collected, only the salt. For example, the Kara Bogaz Gol Bay in the Caspian Sea is a shallow area where the water evaporates under the hot sun and new water comes from the open sea. The salt accumulates in the bay and is later collected for use by chemical and pharmaceutical industries.

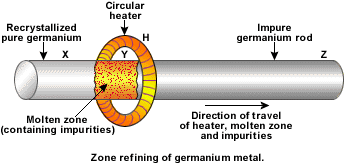
Zone solidification (freezing) is the process where a cold zone moves along a containner with water. The water freezes and then melts again as the zone moves on. The ice in the cold zone separates the initial salty water from the cleaned water obtained by melting the ice. As water freezes, it expels salt, so the ice is virtually free from salt. This process is not typically used on Earth. Instead, another process called zone melting is used for cleaning metals. In zone melting, the hot liquid zone moves along the container. Inside the zone, the metal is liquid. When it gets solid again afterwards, it is clean because, as in the case of water, solidification does not allow many impurities into the solid phase.
Cooling and crystallizing waste is a process more expensive than boiling it for distillation, but on the Moon, with its cold nights, zone solidification would be a natural way to clean water. Once again, kudos to Sasha who suggested using both distillation and zone solidification in turns, distillation during the hot days and zone solidification during the cold nights.
It may appear that using the Moon's outside temperature to boil or freeze water saves energy that would otherwise be needed for heating or cooling. It is not so because we need to use energy to melt frozen water at night and to cool hot vapor during the day.
We tried to test zone solidification experimentally. Unfortunately, the experiment failed. Initially, the plan was to use dry ice to freeze water, but we could not find any dry ice and used overcooled ice from the freezer. The temperature of the ice was not low enough to crystallize water in the test container within the time we had for the experiment. If we are lucky to obtain dry ice, we will will try again.
Sasha was not able to attend this session in person. Nevertheless, she was present virtually, thanks to the miracles of technology .
HOMEWORK ASSIGNMENT:
Please think about the design of the distillation and/or zone crystallization system that would allow a continuos and reasonably simple way to produce fresh water and recycle the residue. If you express your thoughts by the means of a drawing, it would be really great.
The topic of our next class will be experimenting with distillation.
Dear parents, I would highly encourage you to help you children with this assignment.