The Independent Center for Integrative Education: Learning without Limits
01/03/2011
The topic of our second class was the chemistry of water. Please note that there is no homework assignment this time. Everybody deserves a break.
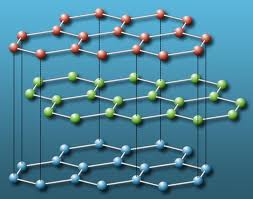
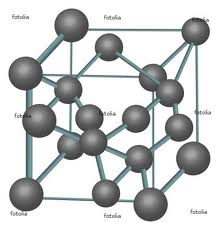
Water is an amazing substance. Not only can it be gaseous (vapor) and liquid, in its solid form there are 11 different types of ice! Those types of ice differ by the pattern of atoms that are packed into crystals, and, of course, they have different properties. This is called allotropism. We discussed another allotropic material, pure carbon (in chemical notation, C). The most common configuration of the atoms forms a hexagon-based structure called graphite (the core of #2 pencils is made of graphite), a black soft substance that can conduct electricity. Under high pressure and high temperature, which could be found in some places underground, the atoms of carbon get arranged differently forming diamond. In an expensive experiment, you can burn diamonds and obtain CO2. Below the structures of graphite and diamond are shown schematically.
 |
 |
Atomic structure of the graphite phase of pure carbon |
Atomic structure of the diamond phase of pure carbon |
Water is also one of very few materials that expand when crystallizing, so that ice is lighter than water and floats on its surface. There are other properties of water that make it unique.
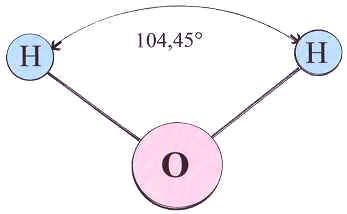
Water is a compound material that consists of molecules with two atoms of hydrogen (H) and one atom of oxygen (O), so its chemical formula is H2O. Counter intuitively, the atoms of hydrogen are not located symmetrically on the two sides of the oxygen atom.
 ;
;
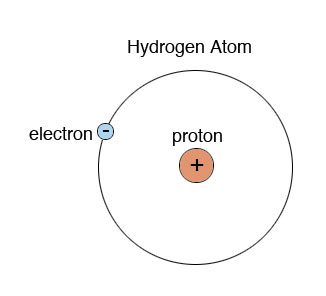
The structure of the hydrogen atom is the simplest of all. Like all atoms, it contains a central core called the nucleus surrounded by a cloud of electrons. In hydrogen, the nucleus contains a single proton and the cloud contains a single electron. The proton is about 2,000 times heavier than the electron, so practically all the mass of the atom is in the nucleus. At the same time, the size of the nucleus relates to the size of the cloud (which defines the size of the whole atom) as the size of an apple relates to the size of Earth. The reason they stay together is that both particles are electrically charged, the proton positively and the electron negatively, and the charges of different signs attract each other. The charges of the electron and the proton are equal except for their sign, so the atom as a whole is electrically neutral.
The structure of the oxygen atom is more complex. The nucleus contains 8 protons and the cloud contains 8 electrons, so the atom is neutral. The similarly charged protons repel each other and the nucleus would break in no time if not for the 8 neutrons, particles of the size and mass of protons but having no electrical charge (hence the name). The neutrons serve as glue that keeps the nucleus together.
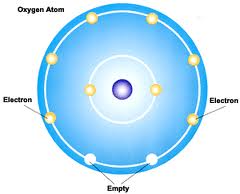
Electronic structure of the atom of oxygen (O)
All electrons cannot sit at the same distance from the atom - they would crowd each other otherwise. According to quantum mechanics, the electrons are arranged in layers. The inmost layer can contain no more than 2 electrons, and the second layer can contain up to 8 electrons. Therefore, hydrogen's outmost layer is half full (or half-empty, depending on your general outlook), and oxygen's outmost layer is almost full, missing just two electrons.
Chemical reactions occur when electrons move from one atom to another, and those electrons naturally are from the outmost layer. The layers that are full are not interested in either losing or gaining electrons. The layer that contains only one electron is prone to lose it or to gain another one, and the layer that is almost full is very much inclined to gain electrons to become complete. So, when to atoms of hydrogen collide, they would share their electrons so that both can consider their outmost layers full, forming a hydrogen molecule H2, a common form of hydrogen gas. When two atoms of oxygen collide, they share their electrons so that their outmost layers feel fuller and form a molecule O2. Atoms with full outmost layers, such as helium (He) do not react to each other (as well as to any other atoms) and do not form molecules. That is why such atoms are called noble gases.
When hydrogen molecules meet oxygen molecules, they find a better arrangement that makes their layers feel even more comfortable. The oxygen atom takes electrons from two atoms of hydrogen and fills out its outmost layer. It makes the oxygen atom negatively charged and hydrogen atoms positively charged, so they stay together. If that was it, the hydrogen atoms would try to stay as far from each other as possible, forming a linear molecule. In reality, however, they form an angle, because they still partially share electrons with oxygen, and quantum mechanics dictates this shape. Therefore, the water molecule, being neutral as a whole, has one side charged positively and the other one negatively.
.jpg)
The chemists use special notation for chemical reactions. The reaction that produces water from oxygen and hydrogen is written as
O2 + 2H2 --> 2H2O
This reaction is also called oxidation or burning of hydrogen and produces so much energy that it can be used to move a car (but if not handled properly, the mixture of oxygen and hydrogen could explode).
In liquid state, the molecules of water are attracted to each other - negatively charged sides to positively charged sides. A hydrogen atom stripped from its single electron can be shared between the molecules forming a so-called H-bond, and many properties of water are explained by formation of this rare type of bond.
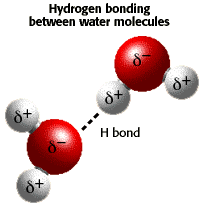
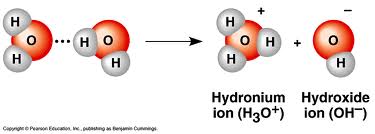
Since the molecules move and oscillate (it is called thermal motion), one molecule would sometimes go away with an extra hydrogen, leaving the other one with only one hydrogen. This reaction, called dissociation, is written as
2H2O --> H3O+ + OH-
The two resulting particles are electrically charged. Charged particles are called ions. In this reaction, two ions are created, a positively charged one called hydronium and a negatively charged one called hydroxide:
It is important, that simultaneously with dissociation, the other reaction occurs, in which two ions meet and recombine back into two molecules of water:
H3O+ + OH- --> 2H2O
When the amount of ions increases, there are more chances they meet and associate back. There is, therefore, some equilibrium amount of the ions when the rates of dissociation and recombination are equal and the concentration of the ions does not change with time. At room temperature, this concentration is 10-7 for each type of ions. The chemists use the degree of the power of ten to characterize this concentration, and call this quality pH, so
for pure water, pH = 7
There are two classes of substances that change the pH of water, namely acids and bases. Let us consider hydrochloric acid, which consists of one atom of chlorine and one atom of hydrogen (chemical formula HCl), as an example. When dissolved in water, each molecule of HCl produces two ions. One of them is a negatively charged chlorine ion with an extra electron that it stole from the hydrogen, and another one is a hydronium ion:
HCl + H2O --> Cl- + H3O+
Therefore, each molecule of the acid dissolved in water adds a hydronium ion, so the concentration of those ions grows significantly. The value of pH decreases with increase of the concentration of hydronium and becomes less then 7. A water solution with pH < 7 is called acidic.
As an example of a base, let us consider sodium hydroxide, chemical formula NaOH. When dissolved in water, it breaks into a positively charged sodium ion and a negatively charged hydroxide ion:
NaOH --> Na+ +OH-
The hydroxide ion recombines with a hydronium ion from dissociation of water, and that reduces the amount of hydronium ions and therefore increases pH:
OH- + H3O --> 2H2O
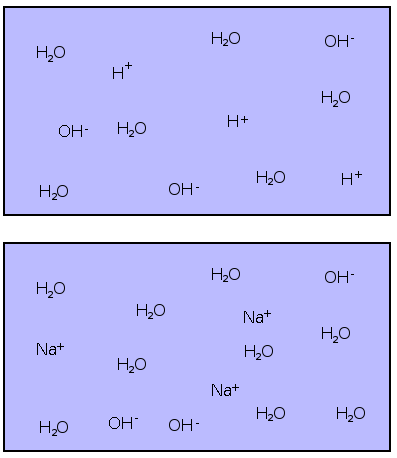
Adding NaOH to water removes H+ ions
Water solutions with pH > 7 are called basic. The diagram below shows pH for different common solutions:
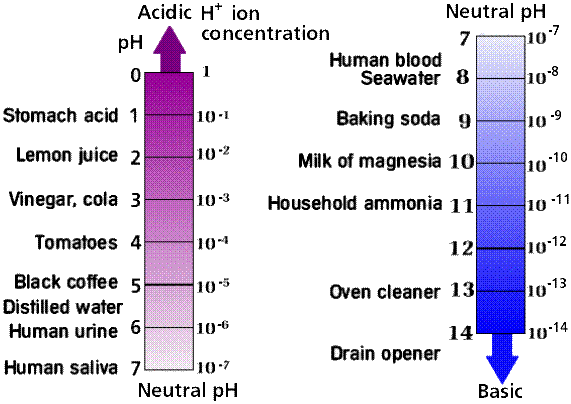
There are chemicals that change their color depending on the pH of the solution. In class, we used one of such chemicals, routinely used by aquarium owners to check the quality of the water. It became blue in a basic solution and yellow in acidic solutions. Using this liquid, we tested some common household solutions for acidity or alkalinity (that is how the strength of the basic solutions is called).
Adding a base to an acidic solution would reduce the acidity and adding an acid to a basic solution would reduce alkalinity. In the right proportion, the acid and the base would totally neutralize each other, bringing pH of the solution to the neutral value of 7:
Na+ + OH- + H3O+ + Cl- = NaCl + 2H2O
We see that such a reaction produces neutral solution of NaCl, sodium chloride, a.k.a. table salt. Of course, the resulting salt is dissolved in water. Removing water from the solution (for example, by evaporation) would produce solid salt.
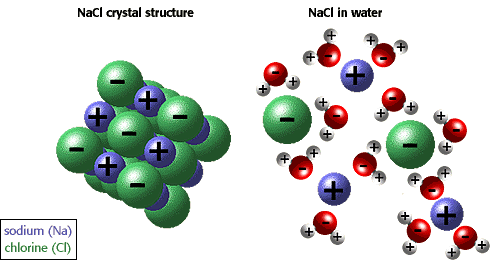
In class, we performed neutralization of vinegar solution with baking soda. We also used special chemicals for conditioning aquarium water for neutralization of acidic or alkaline (basic) solutions.
The topic of our next class will be cleaning water from dissolved salt.