The Independent Center for Integrative Education: Learning without Limits
12/30/2010
The topic of our second class was how and from what water could be cleaned. Please read the summary below and the homework assignment at the bottom.
We agreed, that all kinds of water contamination can be divided into solid particles and liquid chemicals. There are also gases dissolved in water, but if they react with water, like sulfur oxide or nitrogen oxide, they produce liquid acids or other chemicals, and if they don't, like nitrogen or oxygen, they are not really contaminants.
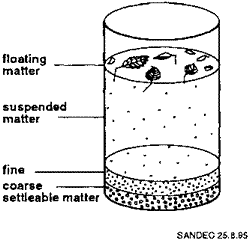
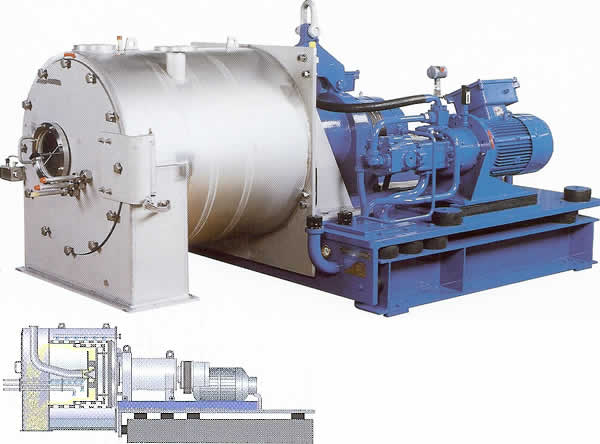
Then we put dirt into a glass of water and saw how large heavy particles sank and light pieces floated, so the middle of the glass was clear of large pieces of dirt. Thus, we saw that we can get rid of large solid pieces just using gravity in the process called sedimentation. The smaller particles take more time to settle down than the larger particles, and some very small particles will always stay because the molecules of water will hit it randomly and prevent from moving in one direction defined by gravity. Even in Earth gravity, the process is slow. It will be six times slower on the Moon. To increase gravity, we will have to use centrifuges, fast-rotating drums where inertia (in a form of so-called centrifugal force) would simulate higher gravity.
SEDIMENTATION |
CENTRIFUGE |
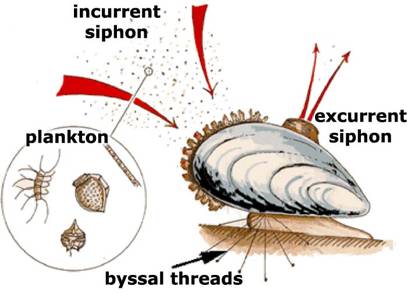
Then we used a paper towel to filter smaller particles. After filtering, water was clear although yellowish. Looking closely, we saw that there were still small particles floating around. If we had filters with smaller holes, we could filter out even smaller particles. There exist filters today that can filter out even viruses. The problem with fine filters is that it takes progressively more time and pressure to seep water through them. Instead, we may consider water-living animals, such as sponges or mussels, which filter water for food.
PLANKTON FILTERING (SKETCH) |
AND HOW IT ACTUALLY HAPPENS |
When it comes to liquid chemicals dissolved in water, we may use other chemicals to fight them. Ideally, we would need to add someting to water to bind contaminats into solid particles or gas bubbles that can be then removed by gravity or filtering. We can also use phase transformations of water, namely evaporation and freezing, to clean it from dissolved contaminants.
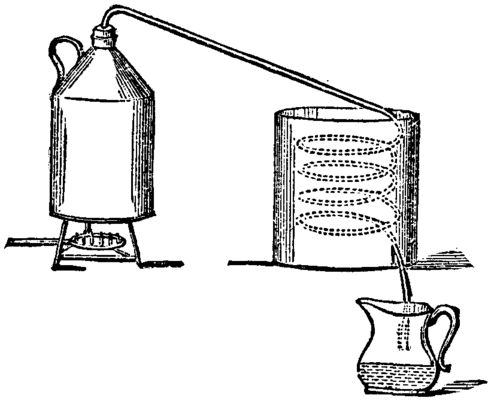
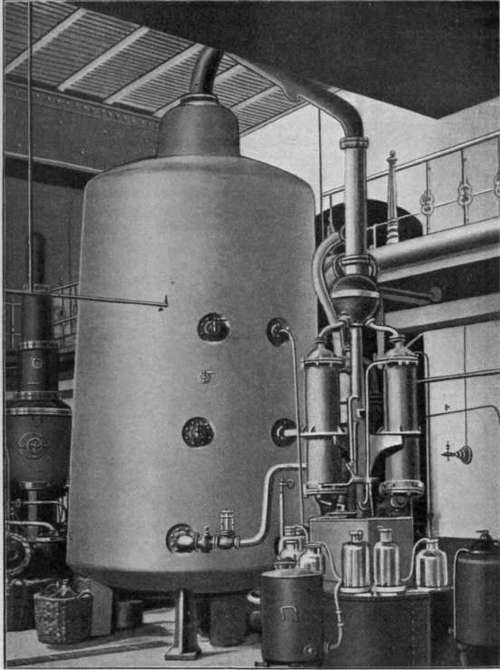
The cleaning process based on evaporation is called distillation. When water evaporates, many solvents (chemicals that are dissolved in water) stay behind. The water vapor then travels in tubes where it eventually cools down enough to condense again. If volatile compounds were dissolved in water, such as alcohol, ammonia, or ether, they would also evaporate but they would condense at different temperatures, so we can collect water at one place and alcohol in another (besides producing liquid water, this process is also used to produce liqueurs).
DISTILLATION PROCESS |
30,000 LITER DISTILLATOR |
DISTILLATION PLANT |
Freezing is another process that can clean water. Water in the ocean contains a lot of salt, but when it freezes, the ice consists of fresh water and is good for drinking when melted. Many other chemical contaminants are left behind when water freezes. Where do all this stuff go? Obviously, when all the water freezes, everything that was in it will still be there. In fact, for real cleaning we need a process called zone refining. We take a long container filled with water, we cool it down at one side, so that water there freezes. The ice is clean and the rest of water contains all the contamination. The more water we freeze, the dirtier the liquid remainder gets. We should stop at some point, otherwise the remaining water will be so dirty that even when it freezes, the ice will be dirty as well. So, we take away the clean ice and are left with even dirtier water than we started. Therefore, by zone refining, we cannot clean all the water, only a part of it.
Sasha suggested an excellent idea that would only work on the Moon and not on Earth. She suggested that during the hot daytime with temperatures much higher that water boiling point we can clean water through distillation and during cold days when temperature is much lower than the water freezing point, we can use the cold for zone refining. We can collect dirty water that remains after zone refining and clean it by distillation during the day. On Earth, zone refining is used with metals and other minerals (for example artificial sapphire). It is not used for water purification, because it would be more expensive than distillation and because frozen sea water is already pure. The situation on the Moon is different by both counts: at night it is naturally cold and the water we need to clean is still liquid.
ZONE REFINING OF METALS |
In the Lunar station, water would come from many different sources. It is relatively easy to collect liquid water (urine, soapy water from washing, etc.) but much water is excreted into the air (when we breathe, we exhale as much water as carbon dioxide) and collecting it would take condensing it first. We will need special condensers similar to dehumidifiers many people keep in their basements. They are, in essence, little refrigerators with fans that cool air enough for water vapor in it to condense.
Figuring out how to clean water is only part of the problem. To clean water, we need to collect it first, and after cleaning we need to find a way to recycle the residue. We cannot just dump it, because what was dissolved or, in the case of solid particles, suspended in the water comes from something that we used and will need to use again. For example, throwing away animal and human waste, we eventually will run out of the stuff from which our plants can produce food. Designing the water recycling system, we need to recycle the residue.
HOMEWORK ASSIGNMENT:
Please read the part of the Educator Guide that discusses water recycling, pages 14 to 21 (the page numbers correspond to those on the pages and not the ones that are shown by your PDF reader). Choose a stage, or stages, of water recycling that feel interesting for you and research them using Google, Wikipedia, and other resources you can think about, so that you will have something interesting to tell us in the next class.
The topic of our next class will be general design of the water recycling system we are going to build..
Dear parents, I would highly encourage you to help you children with this assignment.